Human Biological Specimen Collection for Clinical Research
Does your clinical research require human biological specimen collection? We provide specimen collection services for all types of clinical research.

Our Specimen Collection Procedure
We manage the collection of urine, serum, and other human biological specimens to support your clinical research. Each type of specimen is collected, transported, and stored according to CDC collection guidelines and international research standards.
Our large collection network allows us to offer the most comprehensive specimen collection services. We also offer patient recruitment services and data collection and curation.
1. Patient Preparation
Patient preparation is a crucial step in the sample collection process. We provide patient instructions, including date and time of collection, medication restrictions, fasting guidelines, dietary requirements, and other guidelines appropriate to the specimen type and to the clinical goals.
In addition to providing collection instructions, we also collect the patient’s information, such as patient name, date of birth, address, medical history, billing information (if applicable), and more. A high level of transparency is maintained, from obtaining informed consent for the potential future use of samples, to making research results available.
2. Specimen Collection and Handling
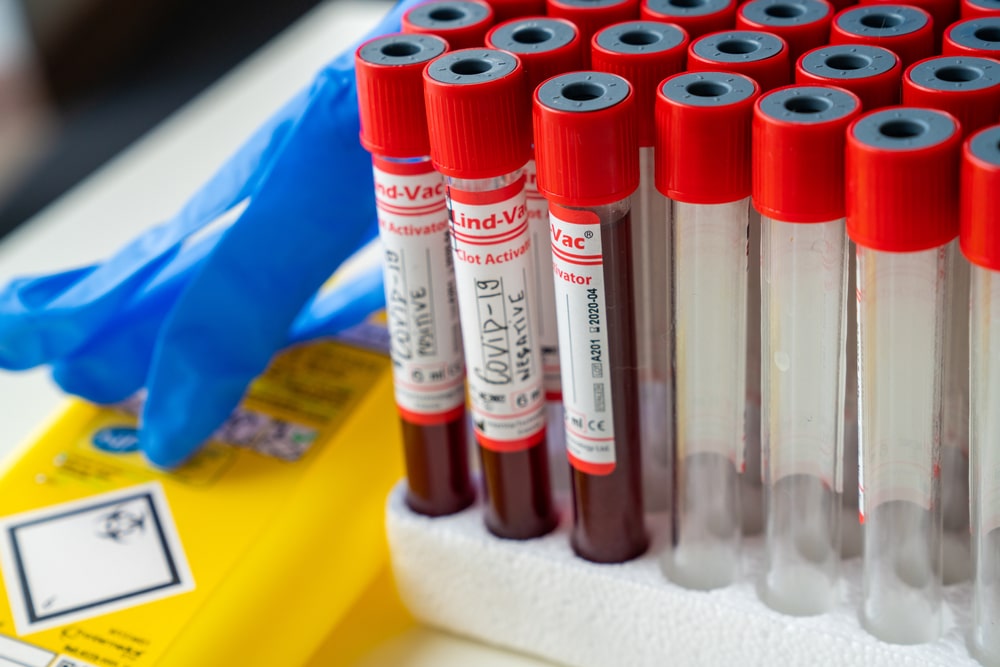
Clinical results are only as good as the biological specimen collected. We use clean, dry specimen containers of the proper type for sample collection and transport, including for COVID-19 testing, surgical pathology, muscle biopsies, or any other clinical purpose.
Whether for stool collection, urine collection, blood collection, or any other specimen types, we check that each sterile specimen container is properly labeled and has the correct patient information.
3. Specimen Transportation and Storage
Immediately after collection, blood specimens and other biological samples must be properly stored and transported to the laboratory immediately to ensure quality clinical outcomes.
Each specimen type requires a specific transport medium and storage mode. We use appropriate precautions and follow international storage guidelines for all types of human specimens, including serological tests, respiratory specimens, urine samples, renal biopsies, and more.
4. Specimen Submission
After collecting, processing, storing, and transporting biological specimens, it’s time to submit them to a participating laboratory for analysis.
Each collection container must be properly labeled and identified to ensure accurate test results. Infiuss partners with health centers and clinical laboratories to ensure the timely analysis and processing of biological research specimens.
Test Request Form Services
Lab testing of biological specimens is a critical component of a successful clinical research.
Upon transporting biological specimens to the laboratory, appropriate test request forms must be in place for each specimen submitted to ensure proper handoff between collection personnel and laboratory personnel. We can help you develop and implement appropriate laboratory test request forms or test requisition forms for laboratory testing of specimens.
Types of Specimen Collection
These are some of the common types of biological specimens collected for clinical research. Each specimen is collected into the appropriate sterile container, processed, stored, and transported according to international standards for that specimen type.
Swab specimens, or upper respiratory samples
Fixed tissue, including formalin-fixed paraffin-embedded (FFPE)
Blood draws
Cerebrospinal fluid
Urine and stool samples
Fresh frozen tissue
A Full Range of Clinical Research Services
Beyond specimen collection, Infiuss is your partner for all clinical research needs. From patient recruitment to clinical data management, pharmacovigilance to site management, we will partner with you for all types of clinical research.
Stay Informed
Find new health insights
Infiuss Health insights contains inspiring thought leadership on health issues and the future of health data management and new research.